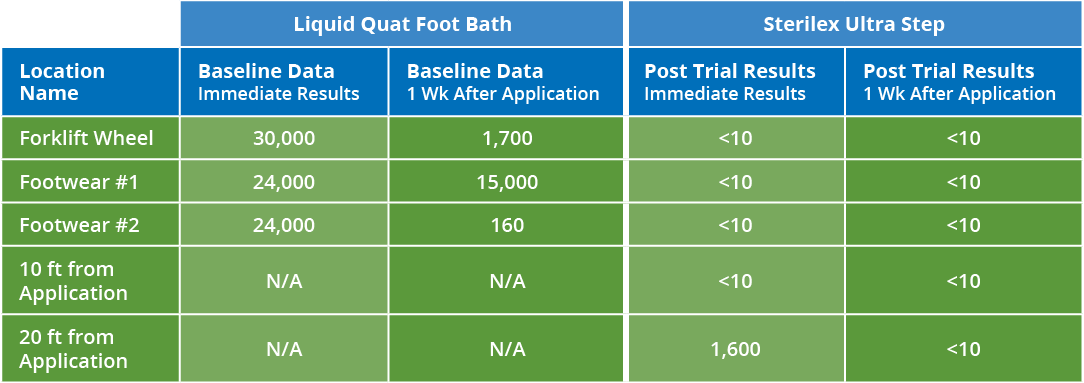
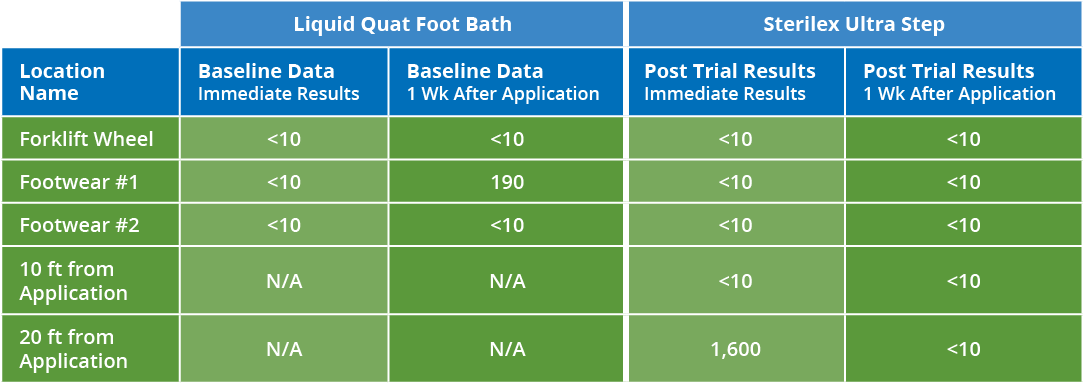
Os pisos de produção são o maior vetor de contaminação cruzada. A desinfecção dos pisos durante a produção permite que as fábricas reduzam a carga microbiana que vai para a higienização e girem as linhas mais rapidamente. Continue lendo para ver as fotos de antes e depois de um planta de café que usaram a tecnologia PerQuat® da Sterilex para reduzir os resultados positivos de patógenos.
O acompanhamento com os higienizadores a seco da Sterilex não só aumenta a tração e ajuda a evitar escorregões, como também acrescenta uma camada adicional de proteção contra a contaminação cruzada. Além disso, o Ultra Step e o ProvaStride da Sterilex podem ser usados em ambientes, como a fábrica em este estudo de caso, onde a umidade é uma preocupação, e não perca nosso white paper, que examina o impacto do desinfetante seco e das intervenções nas portas sobre a tração.